I ‘m been busy doing some aluminum parts, and after you work hard in a part you want it look beautiful and last long. So here is when anodizing comes. Most of the experience here is based on Ron Newman’s Anodizing Aluminum, the best anodizing guide on the net.
A Brief Overview of Anodizing
I don’t now much about chemical reactions, so this overview will be very basic and not fully precise.
Anodizing is a process to colorize and protect aluminum. Through an electrolytic process a fine coating layer of aluminum oxide is grown. This layer has open pores on it, ones that can be filled with color dye and sealed. Aluminum oxide is a very hard material, so though only a few microns depth, this layer protect the part from small scratches and gives it a beautiful and professional finish.
There are at least two anodizing types, depending on the coating thick: Type II (1.8 μm to 25 μm thick), and Type III (thicker than 25 μm) or “hard anodizing”. Hard anodizing obviously is more durable, but also more difficult to do at home, so the anodizing done here will be Type II.
The main steps involving in anodizing aluminum are:
- Clean: remove any grease or dust.
- Desmut: remove smut generated from previous step.
- Coating: create oxide layer
- Dye colorize: fill oxide pores with special dye.
- Sealing: seal pores so the dye stay in the surface.
Every step requires a specific chemical, and time and/or temperature control.
What do you Need
The things do you need to anodize are:
- Some plastic pots.
- A metal pot.
- Distilled water (at least 5 lt).
- A battery charger or power supply of several amps. Note that some chargers have a “auto-shutdown” and can’t be used.
- Current meter (10 Amp at least).
- Caustic soda (lye) solution, the one used to clean pipes.
- Nitric acid solution (10%). I think that this is not really required for 606X aluminum types.
- Sulfuric acid solution (15%).
- Aluminum wire. Mine is 1.5 mm diameter.
- Graduated glass beaker.
- Anodizing sealer. I got “ALSE22 ” from Caswell.
- Anodizing color dyes. I got red, block and brown from Caswell.
- A small balance or method to weight sealer powder.
- Anode. Aluminum foil will suffice.
- Some support to hang the parts over the pot. I build a nice stand for this.
- Electric cable.
- Rubber globes, security glasses, old waring, etc.
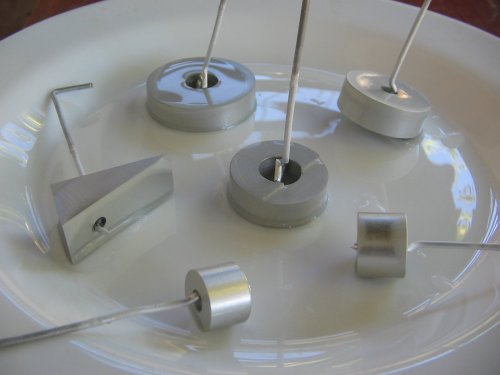
Below is some of this stuff (the funnel was never used).
Here is my stand and anode setup. The holes and screws allow easy mount of the aluminum wire. As can be noted, this pot fits only small pieces (the only ones I can machine).
Mounting a mini-anodizing line
Please note that the chemicals used here are potentially dangerous and that some nasty gases are produced.
First time anodizing will be hard, but once you have a mounted anodizing line and doing some runs, anodizing will be rutine and take only a couple hours. Here is how I build mine:
- Water (small pot). This will be used to clean when passing the pieces between pots.
- Nitric acid solution (small pot): 500 ml watter + 100 ml nitric acid (68%).
- Sulfuric solution (large pot): 1000 ml water + 150 ml sulfuric acid (98%) (NEVER add water to acid, ALWAYS add acid to water).
- Dye solution (large pot): 1000 ml water + 15.6 ml dye.
- Sealer solution (metal pot): 600 ml water + 5 grams sealer.
I store the pots for later reuse, but discard sealer (I’m not sure if can be reused; anyway 1 lb package from Caswell will last).
Area and Time Calculation
In the electrolysis process the oxide coating layer grows up to a maximum thickness; after that, the coating remains the same thickness but the part begins to shrink. Hence electrolysis time is a important parameter. It depends on current and total cathode area. Less time will result in a thinner oxide layer; too much time will result in a smaller part. I use the “720 amp-min per square foot” rule to deduce this simple formula:
Full Thickness Time = (A / 929) *720/ I
Where A is the full cathode area in cm3 and I is the nominal current and the result units are minutes.
There are some area calculator for simple shapes on the net; they can help you to do a rough estimate.
For my first try, the total area was roughly 100 cm2, and with a nominal current of 2.4 Amp this gives 31 minutes.
First Attempt
Of course I will not try to anodize my beloved parts in this attempt. Instead, I machined some scraps of 6061 and 2024. The last is know to not be easily anodizable.
So the procedure is:
- Calculate total area for the cathode.
- Clean the parts to remove dust. Rinse and/or use acetone.
- Cut aluminum wire and make hangs for every part. A good electrical contact is a must for a good anodizing. Also please note that the contact points will not be anodize, hence these should be in a less visible area.
- Put 2 min in lye solution. After this step parts should not be touched and should not stay out the water.
- Put 10 min in nitric acid.
- Put in the electrolysis bath and measure how much current is being draw. Estimate time based on this current.
- After half the required time has been elapsed, measure current again and recalculate time with this current. The current will go up in the process, so this will be a rough average or nominal current. Temperature should be in the 20-23 ºC range. The solution will heat up after a while, but I that shouldn’t be a problem when you don’t do continued runs.
- Heat dye solution to 60º and put parts for 15 to 20 min.
- Boil the sealer solution and put the pieces for 15 to 20 min.
Prior cleaning
I clean the parts with acetone before the lye solution. This is the last time you can touch the parts.
After lye solution
After the lye 6061 parts looks the same. However, 2024 get covered with a blackish smut. As far as I know this is due to the copper content in this alloy type.
The lye solution pot
This is the pot size I use for lye, nitric acid and water.
After the nitric acid
Again, 6061 parts look the same, but in the 2024 ones the smut has vanished. I’m not sure if this step is required for 6061 parts; anyway nitric acid doesn’t eat aluminum, so this will not hurt.
In the electrolysis bath
At the start, the meter measures 2.2 Amp, increasing up to 2.6 after 30 min. So I use a nominal current of 2.4 Amp for the time calculation.
After the electrolisys process
After this process, 2024 get darked as the parts in Ron Newman’s info; but 6061 remains almost the same. This lead me to make a mistake. I once read that 2024 anodizes faster than 6061, so I thought most action was by 2024 and 6061 parts get almost nothing coating. Also, I measure the diameters of the 6061 round parts an were the same before the process. So I repeat the process for 6061 parts alone (recalculating time of course). After the elapsed time, the parts looks the same, so something was wrong I think… I measure again, and they were 0.05 mm less in diameter! Then I realize that the 6061 were already coated, and the the second run only eats the surface.
In the dye solution
For sure the most easy way to heat the dye solution will be use a cup heater, but I don’t have a small enough one to fit my pot. So put my pot in a large metal pot and surround with boiled water. After a while the dye reach 55º, and I put the parts inside. This is bit less that the specified, but enough I think.
After the dye
When the parts left the dye solution, It was clear that something was wrong with the 2024, and that the 6061 don’t get a uniform color. Anyway I decided continue and finish the process.
In the sealing pot
And the final step: boil the seal solution and put the parts 15 to 20 minutes.
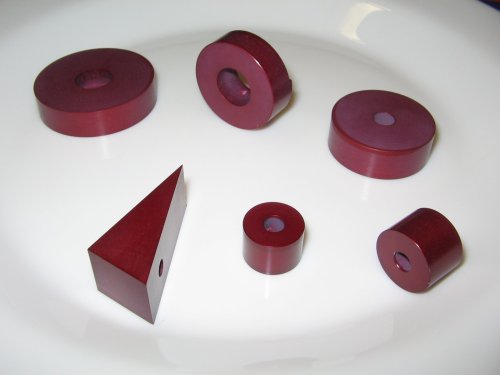
The final result
Well , that’s my first anodizing, far from perfect.
Second Attempt
This is the second try. Here is my improvements and corrections:
- Surface spots were caused, I think, because of time out of the water (or solution). This time I will take the part straight from a pot to another.
- The first time I don’t use pure caustic soda, but a special cleaner… bad idea. May be this cause the small pits.
- I clean the parts in normal water, this time I will always use distilled water.
- 2024 parts were anodized only at the bottom; a bad contact was probably the fail.
- This time I will heat the die to the specified temperature: 60º.
Here an extra step is required: remove the previous anodizing in a lye solution. Please note that the lye solution will get dirty, so don’t use the same you use to clean parts.
After putting the parts in the electrolytic solution, I notice that the current was a bit less that the first time, so adjust time accordingly.
So here is the result. Much better.
After sealing a slight white smut was covering the parts; I successfully remove it from 6061 parts, but remains in the 2024 ones. I think that this was partly due to the fact that after removing previous anodizing 2024 surfaces got a bit porous.
Further
After some anodizing rounds I’ve found that:
- Bad contact is the most common cause of failure. To minimize this, contact points must have some spring capacity. Play with some wire patterns to get something that work. A god wire contact must be able to support the part as this moves.
- A large part loosen at the half of anodizing will be a headache as this will change the required anodizing time.
- No anodizing parts get a dark smut, so after a while they’re easy to identify.
- If dimensional fit is critical for a part, be sure to don’t have to repeat your anodizing.
- Some numbers: 167 cm^2 -> 2.7 Amp; 180 cm^2 -> 3.5 Amp. So I think a 6 Amp supply will suffice for the capacity of the pots I use. These are 15x15x8 cm, and I guess the will support no more than 300 cm^2 of parts.
- I try to use a standard PC supply, but the one I used was cheap, so 12 volts were 10 at 2 amps or so. I think I will buy a standard 13.8 volt – 6 Amp supply.
- To much parts can be trouble to handle, so splitting in groups will be a more secure way.
- The metal pot should have the at least the same deep as the plastic pots; mine is lower an that is a problem. Parts should not touch the bottom of the pot and shouldn’t be near the surface (as the water level lowers).
- Without care, this anodizing will not stay too long without small pits. Though more hard than aluminum, due to its small width, anodizing layer will peel after some rub or shock.
That’s all. If you want to learn how to anodize I encourage you to visit Ron Newman’s Anodizing Aluminum among other resources.













hi!
I like what you have done. I’m trying to get a power supply that would be enough to anno 100-200cm2 parts. I was wondering if 15V 5A labolatory supply would do? my email rogalxxx(a-t)o2.pl
Well, nominal voltage of battery chagers and related power supplies is 13.8v, so I think 15 volts would do the work, may be lowering time by 10% or something.
If you has a micrometer and a test part you can do this check: begin anodizing and stop at regular intervals to check the size the part. If at some point the size diminishes, turn off the power. This way you can sure you had not overcalculated the required time too much.
Also, please note that current draw of a specific setup depends on both catode and anode area, among other things.
Here is great info about the whole process: http://www.rapidfirepaintball.ca/Anodizing.htm
How have you calculated that 500ml of water + 100ml of nitric acid gives you 68%? Is there a formula?
If you take 100ml-68% of nitric acid, you have 68ml of acid and 32ml of water. Now if you add 500ml of water, you get a concentration of 68 / (500+32) = 12,7 %. So 1 part of acid + 5 of water roughly gives a 10% solution.
So basically I should mix 1to 5? One more question. I will do the anodizing process in my garage in which during the winter time its very cold. Do I need to heat up the sulfuric acid solution even if it’s getting warm during the process?
If your acid it’s 68% or near, yes, a 1 to 5 mix will work. I’m not an expert, but I suppose heating it’s not required. Good look with your anodizing!.
Keep in mind that temperature affects how conductive the sodium hydroxide (lye) solution is. The warmer the solution, the more current it will cary. This is why metalAddict observes current changes from start to finish. Preheating the bath will help level the current differences between start and finish
Be extremely careful with the nitric acid . Use it only at well ventilated area. Not all aluminum alloys needs nitric acid to deox , only alloys that contains copper. At 6061 it’s not so critical to deox , it contains very small amount of other metals. Your anodizing bath mast be between 68F to 72F. In a new bath you need up to 15 volts to draw the correct amount of Amps for full anodizing. After a few baths you can use lower voltage. If you don’t have 15 Volt power supply let your cathodes in the acid at least for a week before use it. Don’t mix parts with deferent surfaces , some parts will be anodized some others not.
Thanks you very much for your tips, I will apply them the next time.
wow! super!
Here´s a video that we made for school about the process, you can also see the finished result against the original. https://www.youtube.com/watch?v=SAKLCvEXRyc&feature=youtu.be